What contributes to red wine color? A primer on pigmentation in red wine.
By: Denise M. Gardner
Chemical Structure Figures By: Marlena Sheridan
Red wine color stability has been an important issue identified by the Pennsylvania wine industry during the 2013 and 2014 harvest seasons. It is likely to be a continuing issue for years to come. However, it should be noted that color stability issues are not localized to the Pennsylvania region.

Figure 1: Glass on left contains Chambourcin with deep color intensity while the glass on the right is Chambourcin wine with medium color intensity. Vintage 2013.
To comprehend problems affiliated with red wine color stability, an understanding of the components that are currently known to contribute to red wine color is essential.
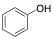
The phenol, a single benzene ring, is the chemical building block that contributes to many components affiliated in wine. Chemically, it is characterized by a hydroxyl group (-OH) attached to a benzene ring.
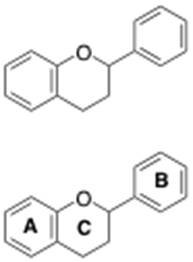
Two classes of phenolics, flavonoids and non-flavonoids, contribute to wine color. For the purpose of this discussion, we will focus on flavonoids, which are defined by containing 3 phenolic rings in their chemical structure, shown below.
There are several sub-classes within the flavonoid class that contribute to red wine pigmentation. This review will focus on 3 of these subclasses:
- Anthocyanins
- Flavan-3-ols (Catechins)
- Tannins (Proanthocyanidins)
Each one of these structures will contain the 3-phenolic ringed flavonoid backbone in their chemical structure (Figure 3). Variation of these subclasses exists with regards to attached units to the flavonoid backbone.
When reading the literature, understanding phenolic chemistry can become confusing, as many words like “tannins” can be used interchangeably with other terms (such as “proanthocyanidins,” “condensed tannins,” or “anthocyanogens”). This can be a daunting challenge for winemakers that are not proficient in chemistry-based terminology. Asking wine chemists for an explanation or clarification of terms is perfectly acceptable.
Monomeric Anthocyanins
Anthocyanins are the red pigment compounds, or chemical structures, that primarily contribute to wine color. Actually, anthocyanins can reflect a number of red-blue hues, often dependent on pH. There are many types of anthocyanins that are identified in grapes (e.g. malvidin, cyanidin, etc.) that are also present in other fruits. It’s important to note that much of the research to date is focused on malvidin-3-glucoside as it is the anthocyanin in highest concentration in Vitis vinifera species (with exception to Pinot Noir) and most easily accessible in a pure form, which makes it easy to obtain for research purposes.
However, many French-American hybrid varieties and native varieties (like Concord) typically contain a different anthocyanin profile in which malvidin-3,5-diglucoside may not be the primary anthocyanin (Ribéreau-Gayon 1959). Many hybrids and native varieties have a higher concentration of other diglucosides or acylated anthocyanins (Hrazdina and Franzese 1974, Ingalsbe et al. 1963). Acylated anthocyanins are often recognized for their contribution to “hybrid red wine character” as their expressed color falls in an orange to light red/pinkish hue (Mansfield 2015). [Note: the term “diglucoside” indicates that there are 2 (di-) sugar (-glucoside) units attached to the malvidin anthocyanin.]
Monomeric anthocyanins, or “free” anthocyanins, are noted for their susceptibility to bleaching by sulfur dioxide or to a change in hue due to a shift in pH. At wine pH (~3.4), only 10% of the monomeric anthocyanins exist in a state that expresses red color (i.e. the flavylium ion) (Somers and Evans 1974). Research has found that the majority of the monomeric anthocyanin exists in the hemiacetal structure, which is actually colorless (Kennedy et al. 2006). This is why anthocyanin concentration does not exactly reflect color intensity or color density of the wine.
Anthocyanin extraction occurs early in fermentation (Kennedy et al. 2006), and is usually at its maximum concentration by day 4 into primary fermentation (Mansfield 2015). Throughout the duration of primary fermentation, the concentration of monomeric anthocyanins will decrease by 10-20% due to insolubility in ethanol and adsorption by wine yeasts/lees (Kennedy et al. 2006).
Flavan-3-ol Monomers
Flavan-3-ols, also known as catechins, are found primarily in grape seeds (Kennedy et al. 2006). These components are large contributors to wine bitterness, but may also contribute to astringency at a lesser extent (Kennedy et al. 2006). In contrast to anthocyanins, monomeric flavan-3-ol concentrations tend to increase throughout the duration of primary fermentation (Kennedy et al. 2006). This is predominantly due to the increased allowable extraction time as seeds are in contact with the ethanol environment.
Tannins
Tannins, or proanthocyanidins, are polymers of favan-3-ol monomeric units. Tannins are responsible for the wine’s astringency, and are extracted from grape skins, seeds, and green matter (i.e. stems) (Kennedy et al. 2006). Tannins can also develop through the age of the wine by polymerizing monomeric favan-3-ols. Tannin extraction directly from the grape matter also appears to increase with longer skin/seed contact time during primary fermentation.
However, tannin extraction and utilization may also be variable in non-vinifera varieties. A recent study by Springer and Sacks (2014) from Cornell University found that many hybrid varieties actually contain higher concentrations of tannin-binding proteins inherent to hybrid variety’s disease- and cold-hardiness resistance. This could explain limited tannin extraction capabilities often affiliated with hybrid red wines. Additionally, if those proteins remain in the wine through primary fermentation, exogenous tannin additions may immediately bind to those proteins. Protein binding to exogenous tannins may minimize their chemical or sensory impact on hybrid wines.
Color Pigment Complexing: Copigmentation
Copigmentation describes an interaction between an anthocyanin and non-colored substrate (often referred to as a “copigment”) that enhances red-blue color hues of young red wines (Boulton 2001). Copigmented complexes are linked non-covalently. I like to think of this as the human equivalent of two individuals linking arms: the unit of 2 can still act as one, but can also easily separate.
The non-colored substrates affiliated with copigmented complexes include monomeric phenols (Figure 2), cinnamic acids, quercitin glycosides, vitexin, and orientin (Boulton 2001). Tannins play an extremely minor role in copigmentation (Boulton 2001).
It should be noted that some white grape varieties are believed to contain higher concentrations of non-colored substrates compared to many red wine varieties (Boulton 2001). Therefore, the act of blending 10-15% of a white grape variety (or white grape skins and seeds) to a red wine fermentation is believed to enhance the potential for copigmentation formation. Essentially, the concentration of co-factors is increased to encourage substrate availability for copigmentation. Malvasia with Sangiovese or Viognier with Syrah are classic white-red fermentation blends used in Old World winemaking regions to enhance red wine color. This phenomenon does not work if the white variety has insufficient concentrations of non-colored substrates (Boulton 2001).
Copigmented complexes are mainly affiliated with young red wines, as they tend to cause a shift in hue from red to a purplish-red. These complexes are less susceptible to sulfur dioxide bleaching or changes in wine pH compared to monomeric anthocyanins.
Color Pigment Complexing: Polymeric Pigments
If the human equivalent of copigmentation complexes are two individuals linking arms, polymeric pigments can be thought of as two individuals each contributing one their individual legs into one pair of pants. This is because polymeric pigments are covalently linked anthocyanin-flavonoid complexes. (Recall that the term “flavonoid” can account for many other things such as another anthocyanin, tannin, etc.) Polymeric anthocyanin-anthocyanin pigments are known as vitisins (Kennedy et al. 2006).
Polymeric pigments can form under two different mechanisms. The first is through a direct linkage of an anthocyanin and another flavonoid substrate (Figures 4 and 5). In this case, the two units are covalently linked together. The image below details this using chemical structures and a schematic.
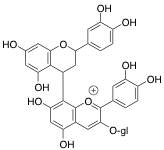
Figure 4: Chemical Structure Showing Direct Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation

Figure 5: Schematic Picture Emphasizing Direct Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation
The second form of polymeric pigments is through acetaldehyde-bridged complexes (Figures 6 and 7). In this situation, acetaldehyde, an aroma compound typically associated with wine oxidation, forms a link, or bridge, between the anthocyanin and another flavonoid substrate. This is detailed below using chemical structures and a schematic.
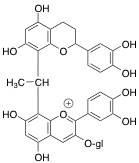
Figure 6: Chemical Structure Showing Indirect Acetaldehyde-Bridged Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation

Figure 7: Schematic Picturing Indirect Acetaldehyde-Bridged Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation
To make this topic even more confusing, the phenolic rings affiliated with anthocyanins and/or tannins can open and re-arrange within the polymeric pigment formation to form what is known as pyranoanthocyanins.
The advantage of polymeric pigment formation is that these complexes are not susceptible to sulfur dioxide bleaching or changes in wine pH. They are the most stable form of wine color known to scientists.
However, polymeric pigment formation tends to occur slowly in wine. Increased extraction of anthocyanins and tannins during primary fermentation offers available substrates to encourage polymeric pigment formation. However, increased extraction does not necessarily guarantee increased polymeric pigment formation.
Many winemakers add exogenous tannins at the beginning of primary fermentation in an effort to increase availability of tannins (or flavonoid substrates) when the anthocyanin concentration is highest. [Recall, anthocyanin concentration will decrease 10-20% by the end of primary fermentation.] This action is often referred to as the addition of “sacrificial tannins.”
However, not all exogenous tannins are created equally, or chemically react the same way in all wines. Additionally, many exogenous tannin products are not in a pure state (Mansfield 2015). Dr. Anna Katharine Mansfield recently reviewed phenolic wine chemistry and this concept in a 2015 Wines and Vine’s article titled: A Few Truths About Phenolics. For a more comprehensive understanding of phenolic chemistry, tannin structure (hydrolysed versus condensed tannins), and color stability, this article offers an easy read for any winemaker.
For winemakers looking to improve the color stability of hybrid varieties, remember that Springer and Sacks (2014) recently determined the high tannin-binding nature of many red hybrid varieties. This may indicate that tannin additions to red hybrid wines may not be useful.
Furthermore, lower anthocyanin concentrations (<200-300 mg/L catechin equivalents) may not be enough to drive the polymeric pigment reaction forward (i.e. create more polymeric pigments). A recent study by Sheridan and Elias (2015) investigate the polymeric pigment potential when acetaldehyde concentrations were increased during primary fermentation.
In order for polymeric pigment formation to occur, all three substrates are required at optimal concentrations (not clearly defined) of anthocyanins, other flavonoid units/tannins, and potentially acetaldehyde. This point alone may be a valuable indication for industry members to retain analytical records that measure the phenolic chemistry of incoming fruit and wines that have recently finished primary fermentation (prior to malolactic fermentation). Such records can act as indicators in terms of what further production methods may not be beneficial for red wine color enhancement. For example, a wine that is deficient in anthocyanin content (<200 mg/L) may not be a wine suitable for things like micro-oxygenation techniques that may contribute to polymeric pigment formation, but may benefit from potential co-fermentations with white varieties to enhance copigmented complexes.
In a future blog posts, we will explore the acetaldehyde-driven polymeric pigment reaction and how it influences red wine color stability. Additionally, this will be supplemented with a research-based literature review of common winemaking practices (e.g. cold soak, extended maceration, delayed MLF, etc.) that are commonly believed to enhance red wine color stability.
Literature Cited
Boulton, R. 2001. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 52(2): 67-87.
Kennedy, J.A., C. Saucier, and Y. Glories. 2006. Grape and wine phenolics: history and perspective. Am. J. Enol. Vitic. 57(3): 239-248.
Mansfield. A.K. January 2015. A few truths about phenolics. Wines & Vines.
Ribéreau-Gayon, P. 1959. Recherches sur les anthocyannes de vegetaux. Application au genre Vitis. Librairie general de l’enseignement, Pairs.
Sheridan, M.K. and R.J. Elias. 2015. Exogenous acetaldehyde as a tool for modulating wine color and astringency during fermentation. Food Chem. 177:17-22.
Springer, L.F. and G.L. Sacks. 2014. Protein-precipitable tannin in wines from Vitis vinifera and interspecific hybrid grapes (Vitis spp.): Differences in concentration, extractability, and cell wall binding. J. Agric. Food Chem. 62(30):7515-7523.
Somers, T.C., and M.E. Evans. 1974. Wine quality: Correlations with colour density and anthocyanin equilibria in a group of young red wines. J. Sci. Fd. Agric. 25:1369-1379.