Tasting Chambourcin: Part I
By: Denise M. Gardner
Note: Sensory descriptions of wines produced by the grape variety, Chambourcin, are based on individual observations, tastings, and a collection of notes obtained through various Chambourcin tastings including many different individuals.
At the Central Pennsylvania regional winery meeting held at Brookmere Winery, attendees and I had the opportunity to taste through a series of Pennsylvania-grown and produced Chambourcin wines. This was actually one of the first all-Chambourcin wine flights that I have been able to taste, and I was quite encouraged by what I was tasting in the glass. Paula Vigna, writer for The Wine Classroom via Penn Live, has since written an article on the tasting titled, “Chambourcin’s ceiling: Maybe higher than originally thought.”
Chambourcin: A Description
Chambourcin is a French-American hybrid wine grape variety that was bred by crossing Seyve-Villard 12-417 (Seibel 6468 x Subéreux) with Chancellor*, commercialized in 1963 (Robinson et al. 2012). Despite Chambourcin’s vigor and relative tolerance to disease pressure in humid climates, anecdotally the wine does often appear preferred by many Vitis vinifera winemakers.
As a wine, Chambourcin’s strength is its vibrant red color and supple, soft mouthfeel due it is relatively lack of course tannin on the palate. These features often make it a valuable red wine blending possibility, especially considering the relative consistency of obtaining Chambourcin fruit every vintage. However, the smoothness of the wine often is a frustration by many eastern winemakers looking for more depth and [tannin-related] mouthfeel in their red wines. When coupled with Chambourcin’s notorious ability to retain acidity, often above 7 g/L of tartaric acid (depending on where and how it is grown), the lack of perceived tannin can make the wine taste relatively thin and acidic.
The acidity associated with the Chambourcin grape variety often appears retained when grown in cooler climates. For example, in Pennsylvania, Chambourcin produced in North East, PA (Erie County) often has relatively higher TAs compared to Chambourcin grown in southeastern, PA (e.g., Berks County). From a grape growing perspective, all winemakers should expect this phenomenon. However, Chambourcin can retain a higher acidity even when grown in the warmer southern parts of Pennsylvania. Based on observation, the variety seems to maintain its acidity when it is not thoroughly crop thinned. As Chambourcin is an incredibly vigorous variety, and as you will see from the tasting, producers hoping to drop the acidity often crop thin grape clusters while on the vine.
When looking at the tannic composition of Chambourcin, it is likely that much of the tannin content associated with Chambourcin is lost during primary fermentation. Dr. Gavin Sacks at Cornell University is studying this situation associated with many hybrid wine fermentations. As Dr. Sacks discussed at the 2016 Pennsylvania Wine Marketing & Research Board (PA WMRB) Symposium in March, tannins come from 3 different components of the grape: the stems, the skin, and the grape seeds. During the fermentation process, anthocyanins (red pigments) and skin tannin is extracted quickly, usually before the product starts to ferment. Seed tannin is extracted more slowly, typically throughout primary fermentation and extended maceration processes. Dr. Sacks’ lab (Springer and Sacks, 2014) and previous research (Harbertson et al. 2008) have shown, grapes produced outside of the western U.S. generally have lower concentrations of tannin available in the grape. While available tannin in the grapes does not necessarily correlate with tannin concentrations in the finished wine, many eastern U.S. winemakers will add exogenous tannin pre-fermentation, during fermentation, and/or post-fermentation to help improve mouthfeel and potentially increase substrate availability for color stability reactions. However, even with exogenous tannin additions, Dr. Sacks has found that many of the tannins associated with hybrid fermentations end up lost during the fermentation process due to protein-tannin binding complexes that pull tannins out of the wine. The higher protein concentration associated with the hybrid grapes has been linked to disease resistance mechanisms that the varieties were originally bred for, and is more thoroughly explained in Dr. Anna Katharine Mansfield’s recent Wines & Vines article, “A Few Truths About Phenolics.”
Additionally, Chambourcin has been noted to have a relatively neutral red wine flavor, lacking a concentrated pop of fruit and using non-descript aromatic or flavor descriptors like: red cherries, red fruit, red berries, stemmy, herbal, or even millipedes. And yes, I have heard one or two consumers actually reference a millipede aroma when tasting Chambourcin.
Tasting Chambourcin Produced in PA

Figure 1: Flight of Chambourcin Wines tasted at the June 2016 Central PA Regional Wine Meeting, hosted by Brookmere Winery. Photo by: Denise M. Gardner
The official flight (Figure 1) of Chambourcin wines that I had put together included:
- Galen Glen Winery 2014 Stone Cellar Chambourcin
- Penns Woods Winery 2014 Chambourcin Reserve
- Vynecrest Winery 2014 Chambourcin
- Pinnacle Ridge Winery 2013 Chambourcin Researve
- Allegro Winery 2012 Chambourcin
Additionally, Brookmere Winery, Armstrong Valley Winery, and Caret Cellars (Virginia) added Chambourcin wines to taste. The formal wine tasting turned out to be quite a unique experience.
I saw a few over-arching sensory themes within these wines:
- Reduced acidity: While I did not personally measure the pH and TA for these wines, the perception of acidity was not as obvious, overly perceptible, or offensive.
- Soft, supple mouthfeel: Even with a couple of the wines that were perceived as “more tannic,” these wines were soft and easy-drinking.
- Use of oak barrels: Many of the producers were opting for some production in actual oak barrels as opposed to using oak alternatives. Type of oak ranged from French, American, and Hungarian.
- Higher alcohols: Alcohol concentrations for these wines ranged from 13-14%, likely due to extended hang time in the vineyard, allowing for an increase in sugar accumulation.
- Two emerging aromatic profiles: A couple of the wines were very fruit-forward and fruitier than what is normally expected from Chambourcin. The other wines were less fruit-forward, however, they did retain a fair amount of red fruit aromatics in addition to the complex aroma nuances: earthiness, tobacco, toasted oak, vanilla, and tobacco. Many tasters commented on the general concentration of aromatic nuance associated with many of the wines we tasted.
In general, the relative depth, cleanness, and fruit expression of these wines was impressive. Perhaps this tasting clearly indicated that although many winemakers struggle with finding the “right fit” or style for their Chambourcin, the level of quality associated with the wine has definitely improved within the last 5 years. At the very least, the level of quality associated with this flight of wines was encouraging for hybrid red wine producers.
Additionally, Brookmere Winery provided a real treat from their cellar library: a 1998 Chambourcin produced by Brookmere Winery (Figure 2) when Don Chapman owned the winery. If you appreciate older wines, then this Chambourcin would truly impress you. Not only did it express the “old wine” honey-floral character loved by many wine enthusiasts, but the red fruit aromas and flavors were still boldly expressed in the wine. The color was intense and dynamically red, and there was a fine perception of firm tannins on the palate. Overall, the tasting of this wine gave me the perception that not only did this wine still have plenty of room to continue aging in the cellar, but that Chambourcin, as a wine varietal, had positive potential for aging for more than 10 years.
Given that most of this post is based on my own experiences, perceptions, and information gathered from growers and producers pertaining to Chambourcin, I would welcome any additional experiences with the variety (as a grape or as a wine) in the comments section.
While this post has documented Chambourcin as a grape variety and a small snap shot of sensory perceptions from a handful of producers in Pennsylvania, next week’s post will focus on production techniques to improve the quality Chambourcin red wines.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
For more information on upcoming regional meetings and the types of tastings to be held at those FREE events, please visit: http://extension.psu.edu/food/enology/events
- Northwestern PA (Erie County) – Individual Site Visits
- Southwestern PA (La Casa Narcisi Winery) – Talk on High Potassium Winemaking
- Southeastern PA (Vineyard at Grandview) on Thursday, July 28th – Berry Sensory Training hosted by Lallemand, with international wine consultant Dominique Deltei (Must register with Denise (dxg241@psu.edu) as space is limited to 15 people.)
Resources
Harbertson, J.F., R.E. Hodgins, L.N. Thurston, L.J. Schaffer, M.S. Reid, J.L. Landon, C.F. Ross, and D.O. Adams. 2008. Variability of tannin concentration in red wines. Am. J. Enol. Vitic. 59:210-214.
Mansfield, A.K. January 2015. A few truths about phenolics. Wines & Vines.
Robinson, J., J. Harding, and J. Vouillamoz. 2012. “Chambourcin.” pg. 218-219. Wine Grapes. ISBN: 978-0-06-220636-7
Springer, L.F. and G.L. Sacks. 2014. Protein-precipitable tannin in wines from Vitis vinifera and interspecific hybrid grapes (Vitis ssp.): differences in concentration, extractability, and cell wall binding. J. Agric. Food Chem. 62(30):7515-7523.
*Authors Note: Since the publication of this article, a few growers and grape breeders have alluded to the improperly reported parentage of Chambourcin. While it is generally reported and cited as such, it is understood among some wine grape experts that Chancellor is not likely a parent to Chambourcin. For more information on determining parentage of given grape cultivars, please refer to: http://www.vivc.de and search the cultivar name of interest.
Reflections: Winemaking at Penn State
By: Denise M. Gardner
I can officially say that I have now been involved with 5 harvests here at Penn State, with my first harvest in 2011. Returning to Pennsylvania from California in 2011 could not have been a greater challenge to an incoming newbie, and I think it will forever be one of the most difficult vintages I have had the experience to deal with to date. Not only did I manage to lose an entire lot of finished wine down the drain (long story…), but I recognized the need to bring PA-produced research wines to Pennsylvania’s growing wine industry during a daunting season from a weather perspective. Additionally, I saw an opportunity to educate students on how to make wine while they helped me process fruit from the research vineyards. In that first year, 5 lucky college seniors helped me process about 8 different varieties from the NE-1020 “multi-state evaluation of wine grape cultivars and clones” project, which was being financially supported by a multi-state SCRI grant.
Looking back today, I now see that the 2011 vintage provided me with a starting point to work with students and a series of winemaking lessons for future vintages that I continue to recall even today.
I was actually one of those bright-eyed students back in my younger days. I stumbled upon Penn State Extension and Mark Chien by pestering local Extension educators on how to grow grapevines. I still recall the many opportunities Mark, specifically, provided for me despite my age or lack of wine knowledge. Mark taught me how to plant a vineyard, from site selection to digging holes for a trellis, how to monitor vine growth through proper pruning techniques, how to ferment grapes into wine, and the various stages involved in production that went beyond the basic texts on how to make wine. I connected with industry members and was awarded an experience to intern at Lallemand in Toulouse, France before I reached my freshman year in college.

Figure 1: Extension Enologist, Denise Gardner, developed an interest in wine grape growing and production throughout high school. Photos, from left to right, include an annual fermentation lesson during a high school agriculture class, building a trellis system at the local high school, grape vines after 2 years of growth at the high school vineyard, and a lesson from past Extension Viticulturist, Mark Chien, on how to properly prune grapevines at a PA vineyard. Photos provided by: Denise M. Gardner
When I arrived to Penn State in 2011, I had a memory of the opportunities Extension awarded me and a goal of working with students that may have an interest in wine production. I still laugh when I recall a number of students that experienced a harvest at a local winery, only to tell me it was “the hardest thing they have ever had to do.”
While many may not make the connection, food science offers an incredible foundation of knowledge that is beneficial for winemakers and those whom wish to go into fermented beverage production. Students engage in a series of classes to develop a foundation in chemistry, microbiology, and biotechnology. Additionally, they learn important processing parameters that are affiliated with winemaking: sanitation, quality control practices, safety when processing, proper sampling techniques, and experimental practices to improve food/beverage products.
For that reason, the annual harvest and production of wine with use of undergraduate and graduate students’ support has blossomed into many positive ventures:
- Since 2010, Penn State Food Science and several Pennsylvania wineries have sponsored student co-ops at wineries during vintage seasons. These experiences educate students in wine production, and specifically provided venues for “real world” experiences in the wine industry.
- Undergraduate students have embarked on undergraduate research experiences pertaining to wine research within the College of Agricultural Sciences. Several of these projects have benefited the local wine industry.
- Graduates from Food Science have started to “harvest hop” to the southern hemisphere for winemaking and production experiences internationally. Virginia (Smith) Mitchell, head winemaker at Galer Estate Winery, traveled to Australia in the winter months of 2013 while Allie Miller will travel to New Zealand for the 2016 harvest. These experiences bring a global perspective and education that facilitate innovative changes to the growing Pennsylvania wine industry.
- Several students have benefited from permanent placement in the wine and fermented beverage industries upon graduation, and many have committed to Pennsylvania operations. The experience gained through research winemaking here at Penn State is invaluable and leaves them with base knowledge in wine production.
- Many students volunteer for Extension programming, which gives them the opportunity to present research and educational experiences to industry members as well as network with potential employers (you!).
- Graduate research has flourished. Both Dr. Ryan Elias and Dr. Michela Centinari have several graduate students that are working on applied research projects which address winemaker and grower needs reflected in previous industry needs assessments.
- Since 2011, the number of research wines being made has more than quadrupled. Today, we have outgrown the equipment I used in 2011 and outgrown our storage capacity for research wines. The wines produced at Penn State are annually evaluated at regional Extension events.
With such a positive focus on student development and interaction with Pennsylvania’s grape and wine industry, the 2015 vintage was expected to be our best vintage yet!
The 2015 growing season did not leave much hope for Pennsylvania grape growers and winemakers, and I can recall a series of summer meetings in which winemakers from across the state asked me if I was prepared to deal with a lack of fruit and a bunch of rot in our research winemaking curriculum. Luckily, as Michela will reflect upon next week, the season shaped up to be one of the best I have experienced in my time here at Penn State.
For 2015, we recruited 10 interested undergraduate students for the 2015 harvest season to assist with the research harvests and wine production. This is double the quantity of students that typically enroll in an independent study experience associated with enology.
Students participate in regular wine processing operations, which can be seen in Figures 2 – 7: crushing, pressing, monitoring fermentation, and completing wines through malolactic fermentation. Additionally, at the end of the semester, each enrolled student presents on a wine grape variety of interest.
Many students arrive with a genuine interest in fermentation science, or would like to get more experience in food production. Many of them leave the fall semester with future undergraduate research opportunities, internships/co-ops at wineries, or develop an expectation to graduate with permanent placement in the fermented beverage industry.

Figure 2: The undergraduate students start each fall with a review of lab analysis techniques to learn how to properly analyze juice and wine, which comes in handy during the harvest season. Photo by: Denise M. Gardner
Figure 3: Crush is an essential part of the independent study class and graduate student research. Care is taken by the students to ensure that proper sanitation is utilized, accurate yields are measured, and that treatments are adequately separated into replicate fermentations. Photos by: Denise M. Gardner

Figure 4: Prepping inoculums for primary and malolactic fermentations is an important part of what the students learn how to do throughout the semester. Here, Blair and Cara prep hydration nutrient and yeasts for inoculations. Photo by: Denise M. Gardner

Figure 5: Students also learn how to properly inoculate wines for primary fermentation. A: Marielle, Stephanie, Joe, Garrett, Gary and Blair inoculate Riesling wines; Photo by: Denise M. Gardner; B: Denise and Gary inoculate Cabernet Sauvignon musts; Photo by: Marlena Sheridan.
![Figure 6: Racking techniques without a pump. [From left to right] Liv, Maria, and Marielle rack Riesling juice into replicate fermentation carboys. Photo by: Denise M. Gardner](https://psuwineandgrapes.files.wordpress.com/2015/11/racking-for-research.jpg?w=479&h=639)
Figure 6: Racking techniques without a pump. [From left to right] Liv, Maria, and Marielle rack Riesling juice into replicate fermentation carboys. Photo by: Denise M. Gardner
![Figure 7: Pressing [white/rosé] juice or finished [red] wine is always an experience. A: Gary, George, and Garrett press rosé to prepare for overnight settling, B: Stephanie loads the press with crushed white berries, C: Allie fills a carboy of finished red wine, D: Laura, Marlena, Gary, Garrett, and Blair preparing for red wine pressing, and E: Marielle sits in the splash zone for red wine pressing. Photos by: Denise M. Gardner](https://psuwineandgrapes.files.wordpress.com/2015/11/pressing-collage.jpg?w=479&h=467)
Figure 7: Pressing [white/rosé] juice or finished [red] wine is always an experience. A: Gary, George, and Garrett press rosé to prepare for overnight settling, B: Stephanie loads the press with crushed white berries, C: Allie fills a carboy of finished red wine, D: Laura, Marlena, Gary, Garrett, and Blair preparing for red wine pressing, and E: Marielle sits in the splash zone for red wine pressing. Photos by: Denise M. Gardner
As a general reminder, many of these projects are financially supported through the multi-state Grape Wine Quality Eastern U.S. Initiative SCRI grant (which partially funds the NE-1020 variety trial research program), the PA Wine Marketing and Research Board, and the Crouch Fellowship, among other grant agencies.
Here are just a few snap shots that depict everything that we are currently working on for the 2015 harvest season:

Figure 8: This year’s NE-1020 variety trial projects include yeast trials, an evaluation of tartaric acid additions to red wine varieties grown in high potassium vineyard sites (A), and pre-fermentation juice treatments in Vidal Blanc wines (B). Photos by: Denise M. Gardner

Figure 9: The Crouch Fellowship currently supports a project pertaining to the impact of spray-on frost protection products on grape and wine quality. A: Graduate student, Maria Smith, gets ready for a full day of processing after a full day of harvest. B: Marielle and Cara monitor the red wine fermentations through daily punch downs, temperature logs, and Brix measurements. Photos by: Denise M. Gardner

Figure 10: Graduate student, Marlena Sheridan, takes a photo of a cluster representation for her research project on red wine color stability. Photo by: Denise M. Gardner

Figure 11: Graduate student, Laura Homich, enjoys time in the Noiret vineyard collecting berry samples for her research project that focuses on the effect of canopy management practices on rotundone (black pepper flavor) development in Noiret grapes and wine. Photo by: Maria Smith

Figure 12: Graduate student, Gal Kreitman, prepares inoculates on Vidal Blanc in relation to a project on the influence of copper on thiol-containing aroma/flavor compounds. Photo by: Denise M. Gardner

Figure 13: Another full year of research winemaking at Penn State – vintage 2015. Photo by: Denise M. Gardner
To follow all of our annual research harvest activities, please ‘Like’ us on Facebook: www.facebook.com/PennStateExtensionEnology
Winemaking Practices Believed to Affect Red Wine Color Stability
By: Denise M. Gardner
In previous blog posts, we covered an introduction to what anthocyanins (red wine color pigments) are and how they can be stabilized in wine. Additionally, Marlena Sheridan recently discussed current research on acetaldehyde-bridging amongst anthocyanins or anthocyanin and tannin substances in wine.
This post will evaluate several techniques used during red winemaking, and what the scientific literature has found regarding their impact on red wine color stability.
Anthocyanins undergo three main phases during the course of a wine’s life:
- Development in the grape
- Extraction during primary fermentation
- Stabilization through the life of the wine
While vineyard development is an important aspect of color stabilization, as starting anthocyanin concentration will ultimately determine potential processing decisions for winemakers, the following processes are reflective of winemaking techniques believed to affect red wine color stabilization. As you siphon through the various techniques, please remember that there is no one fix-all solution to improve red wine color stability. Wine is a complex matrix, and results may vary from one variety to the next, or from one vintage to the next.
Cold Soak
Cold soak is a pre-fermentation process in which grape must is held at low (≤10°C, ≤50°F) temperatures. There are various ways to execute a cold soak step in wine production:
- Placing a holding vessel (i.e., a macrobin) in a cool, ambient environment
- Use of tank temperature control
- Use of dry ice
Cold soaking grape must is believed to increase anthocyanin extraction pre-fermentation. Increasing anthocyanin extraction would make stabilization reactions more favorable, pushing equilibrium to building stable anthocyanin-complexes through and after fermentation.
However, several studies have investigated the use of cold soak on various red wine varieties. In two Pinot Noir studies (Gerbaux 1993, Feuillat 1996, Sacchi et al. 2005*), detrimental effects to color were found on Burgundian wines when cold soak was used pre-fermentation. Similarly, Heatherbell et al. (1996) found no difference in wine color for those wines that were cold soaked versus the non-cold soaked controls.
A study that tested freezing must, however, had a different impact on the finished wine color. The variety evaluated was Merlot, and musts were frozen with dry ice. An increase in anthocyanin concentration by 50% and increase in overall tannin concentration by 52% was found in finished wines in which grape must had been frozen pre-fermentation, compared to an untreated grape must, control wines (Couasnon 1999, Sacchi et al. 2005*). Freezing may have a greater effect on anthocyanin concentration, as freezing physically causes berry cells to burst and release its contents.
Practical Winemaking Application: Winemakers do not need to utilize a cold soaking step to increase anthocyanin extraction or improve red wine color stability, as most research suggests there is no effect regarding red wine color stability. However, it is important to note that little research has been conducted regarding potential extraction of phenol and/or tannin complexes (including polymeric pigment content) during the cold soak step.
Saignée
Saignée is the French term for “bleed,” and is utilized as a winemaking technique for making rosé wines by “bleeding off” free-run juice from macerated red grapes. While the free-run juice may be used for rosé production, many winemakers utilize the saignée technique to concentrate (increase extraction) of anthocyanins and tannins in the juice that will be made into a finished red wine. Secondary effects may also increase flavor concentration.
In 1972, Singleton found that the use of saignée increased the flavonoid and anothocyanin concentrations in the concentrated red wine, four months post-fermentation. Gerbaux (1993) found slight increases in color (through sensory analysis) and phenolics of young Pinot Noir wines that had been subjected to saignée practices. However, unlike Singleton, Gerbaux’s study did not find increases in anthocyanin concentration. However, Gawel et al. (2001) found initial increases in anthocyanin concentrations at the end of primary fermentation in pressed Syrah wines, but these concentrations were depleted after 6-months post-fermentation. This study did not investigate the fate of monomeric anthocyanins, and depleted concentrations of anthocyanins were likely caused by potential polymerization or adsorption of anthocynanins onto various other wine constituents (e.g. dead yeast cells, tartrates).
Practical Winemaking Application: While the causes of potential increased color stability are unclear, the use of saignée to concentrate red grape must appears to have a positive outcome on the wine’s color stability properties. Currently, there is no evidence that removal of 10% free-run juice is less beneficial than removal of 20%. However, as saignée is a concentration method, if the grapes are of low quality, the winemaker will only concentrate other poor quality components (i.e. off-flavors, excessive tannin, etc.) with use of this technique.
Micro-oxygenation (Micro-ox)
Micro-ox is the “addition of dissolved oxygen at controlled dosage rates at or less than the oxygen uptake rate of wine” (Paul 2002). According to Dykes (2007), typical dosages rates range from 2 – 90 mg of oxygen per liter of wine per month. The utilization of micro-ox is believed to affect the stabilization of red wine color pigments, and therefore requires adequate starting material (i.e. anthocyanins, phenolics/tannins) in order for this method to be effective.
Theoretically, when used between primary fermentation and malolactic fermentation, the integration of dissolved oxygen should provide the chemical constituents needed to initiate polymeric pigment formation of monomeric anthocyanins. Micro-ox is used as a driving force to influence acetaldehyde concentration and influence acetaldehyde-bridged complexes previously described by Marlena Sheridan. One previous study has shown no rise in acetaldehyde contraction by use of micro-ox (Pozo et al. 2010). However, this study did find that wines subjected to micro-ox treatment did have an increased concentration of sulfite-resistant pigments. Many other wine experts have written on the use of micro-ox, and there is a world of scientific literature available regarding its use and outcomes in red wines, with varied guaranteed consensus. Additionally, micro-ox has potential to alter other characteristics of wines, including mouthfeel or flavor, in both positive and negative ways, which is often dependent on the starting wine chemistry. The extent of this summary does not nearly cover the depth of research regarding micro-ox in wine production, and will be tabled for a later date.
Practical Winemaking Application: The use of micro-oxygenation may be a powerful tool to enhance anothocyanin stabilization. Winemakers are encouraged to work with the unit’s supplier or a consultant with micro-oxygenation experience prior to implementing this strategy into processing procedures. Anecdotally, at Penn State, we have noticed little success of micro-oxygenation to improve red wine color when used on wines that have relatively low anothocyanin concentrations (<200 mg/L GAE).
Thermovinification
Thermovinification is a technique that uses a brief heating step to grape must or juice, and subjecting it temperatures above 60°C (140°F). It is believed that thermovinification enhances both extraction and stabilization of anthocyanins.
Auw et al. (1996) found that the use of thermovinification increased the concentration of free, monomeric anthocyanins in both Cabernet Sauvignon and Chambourcin wines. Additionally, this study also found increased concentrations of phenolics in the Chambourcin wine treated with thermovinification pre-fermentation, and a lower phenolic content in Cabernet Sauvignon wines treated with thermovinification. This emphasizes that solutions to improve color stability may vary depending on the grape variety and its heritage (i.e. native variety vs. hybrid vs. V. vinifera). Additionally, Sacchi et al. (2005) also concluded that it is necessary to have some skin contact time with grape skins after thermovinification treatment in order to enhance extraction of anthocyanins.
Practical Winemaking Application: The use of thermovinification to improve red wine color stability may be a practical tool for red wines, especially those red wines with minimal aging requirement. Thermovinification is not recommended for red wines destined for long term aging. It should be noted that thermovinification may alter flavor of the finished wines. A winery should evaluate the potential sensory impact of thermovinification prior to committing all wines to this process.
Extended Maceration
Extended Maceration is the process of allowing skins and seeds in contact with the finished red wine, post-fermentation, for an extended period of time. Various studies researching the effects of extended maceration on red wines have found increased concentrations of tannins, especially tannins from grape seeds. However, this process has not been shown to increase extraction or concentration of anthocyanins.
Auw et al. (1996) found that a thermovinification treatment was more effective at increasing phenolic composition compared to extended maceration for Chambourcin wines, but that for Cabernet Sauvignon wines, extended maceration was more effective at increasing phenolic concentrations. Watson et al. (1994, 1995) also found an increase in flavanol and polymeric pigment extraction in Pinot Noir wines that underwent extended maceration.
Practical Winemaking Application: Extended maceration will not increase free monomeric anthocyanin concentrations. This practice should only be utilized for winemakers wishing to increase tannin-based extractions. For a review on why the effects of extended maceration may be focused on phenolic extraction, please see Dr. Anna Katharine Mansfield’s recently published article, “A Few Truths About Phenolics,” in Wines & Vines.
Delayed Malolactic Fermentation (MLF)
The most current scientific literature on delayed MLF is coming out of Dr. James Osborne’s lab in Oregon. This practice is literally as it sounds: winemakers allow a wine to finish primary fermentation and wait (without addition of sulfur dioxide) for a specific period of time until MLF progresses naturally or is inoculated. This is an anthocyanin stabilization processing technique.
On studies in Pinot Noir, James and Osborne (2014) found that a 200 day delay in MLF was required for wines that went through MLF to reach a similar concentration of polymeric pigment, statistically, as the control wine that did not undergo MLF. It is important to remember that in order to maintain stability of wines not treated with sulfur dioxide, adequate temperature control (i.e. maintaining the cellar at 50-55°F or 10-13°C) will cause polymeric pigment formation reactions to progress slowly, which may influence why so much time is needed in delaying MLF. While increase temperature would increase the rate of polymeric pigment formation reactions, the increase in temperature also puts wines at greater risk for microbial spoilage.
Practical Winemaking Application: The jury is not out on this practice! Although some winemakers swear this practice works, it is important to remember than many wineries do not run “control” treatments for various practices. As there is much vintage-to-vintage variation amongst wines, it is challenging to draw solid conclusions from commercial wineries that do not utilize a control treatment. Currently, the literature states a delay in MLF may effectively improve color, but the time required for that delay may not be practical from a commercial operation standpoint. Additionally, winemakers must maintain effective strategies at monitoring potential spoilage during a vulnerable period of time when the wine is not protected by preservatives (sulfur dioxide).
While this blog post covers several techniques believed to affect red wine color stability, the review article by Sacchi et al. covers several additional topics including: yeast selection, fermentation temperature, the effects of sulfur dioxide, carbonic maceration, the use of pectolytic enzymes, and utilization of pump-overs and punch downs during primary fermentation. The review of the current scientific understanding of these practices (up to research literature published through 2005) can be found in the American Journal of Enology and Viticulture (AJEV).
Sacchi et al. 2005* The following authors (Couasnon 1999, Feuillat 1996, and Gerbaux 1993) were included in the Sacchi et al. 2005 review article. As initial research article was in another language, information regarding these studies was obtained from Sachhi et al. 2005.
Literature Cited
Auw, J.W., V. Blanco, S.F. O’Keefe, and C.A. Sims. 1996. Effect of processing on the phenolics and color of Cabernet Sauvignon, Chambourcin, and Noble wines and juices. Am. J. Enol. Vitic. 47(3):279-286.
Burns, T.R. and J.P. Osborne. 2014. Loss of Pinot Noir wine color and polymeric pigment after malolactic fermentation and potential causes. Am. J. Enol. Vitic.
Couasnon, M.B. 1999. Une nouvelle technique: La maceration préfermentaire à froid-extraction à la neige carbonique. Premiér partie: Résultats oenologiques. Rev. Oenol. 92:26-30.
Dykes, S. 2007. The effect of oxygen dosage rate on chemical and sensory changes occurring during micro-oxygenation of New Zealand red wine. Diss. Food Sci. Univ. of Auckland.
Feuillat, M. 1996. Vinification du Pinot Noir en Bourgogne par maceration préfermentaire à froid. Rev. Oenol. 83:29-31.
Gawel, R., P.G. Iland, P.A. Leske, and C.G. Dunn. 2001. Compositional and sensory differences in Syrah wines following juice run-off prior to fermentation. J. Wine Res. 12(1): 5-18.
Gerbaux, V. 1993. Etude: de quelques conditions de cuvaison susceptibles d’augmenter la composition polyphénolique des vins de Pinot Noir. Rev. Oenol. 69:15-18.
Heatherbell, D., M. Dicey, S. Goldsworthy, and L. Vanhanen. 1996. Effect of cold maceration on the composition, color, and flavor of Pinot Noir wine. In Proceedings of the Fourth International Symposium on Cool Climate Enology and Viticulture. T. Henick-Kling et al. (Eds.), pp. VI: 10-17. New York State Agricultural Experiment Station, Geneva.
Paul, R. 2002. Micro-oxygentation – Where now.” Australian Society of Viticulture and Oenology, Uses of Gases in Winemaking Seminar Proceedings.
Pozo, A.G., I. Arozarena, M.-J. Noriega, M. Navarro, and A. Casp. 2010. Short- and long-term effects of micro-oxygenation treatments on the colour and phenolic composition of a Cabernet Sauvignon wine aged in barrels and/or bottles. Eur. Food Res. Technol. 231: 589-601
Sacchi, K.L., L.F. Bisson, and D.O. Adams. 2005. A review of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 56(3):197-206.
Singleton, V.L. 1972. Effects on red wine quality of removing juice before fermentation to simulate variation in berry size. Am. J. Enol. Vitic. 23:106-113.
Watson, B.T., S.F. Price, H.P. Chen, and M. Valladao. 1994. Pinot Noir processing effects on wine color and phenolics. Abstr. Am. J. Enol. Vitic. 45:471-472.
Watson, B.T., S.F. Price, and M. Valladao. 1995. Effect of fermentation practices on anthocyanin and phenolic composition of Pinot Noir wines. Abstr. Am. J. Enol. Vitic. 46:404.
The Effect of Acetaldehyde on Red Wine Color Stability and Astringency
By: Marlena Sheridan
Acetaldehyde is a compound found in wine that has a profound effect on color stability and astringency. Acetaldehyde reacts directly with red wine tannins and anthocyanins to form polymeric pigments and modified tannins. Denise has already provided a great review of polymeric pigment formation (see her post form March 6, 2015). Here, I’ll be focusing on the reactions involving acetaldehyde and research we’ve completed on the topic.
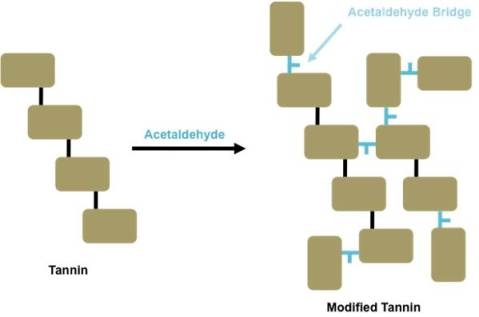
Acetaldehyde reacts with tannins and anthocyanins to form irreversible, covalent bridges. When these reactions are between tannins, they can alter the structure so that its shape and activity are changed. This affects wine astringency, a mechanism based on the interaction of tannins and salivary proteins. Modified tannins, including acetaldehyde-bridged tannins, have been shown to have lower astringency because of their structural changes (Gambuti 2013). A model of these reactions is shown in Figure 1. These changes contribute to the shift from drying or puckering mouthfeel to a more velvety mouthfeel in aged wines.
Similar reactions take place between anthocyanins and tannins as well as between anthocyanins themselves. These reactions form polymeric pigments: pyranoanthocyanins and vitisins (see Denise’s previous post; Cheynier 2006). A model for these reactions is shown in Figure 2. As Denise has covered, these polymeric pigments have increased stability to sulfite bleaching and pH changes compared to monomeric anthocyanins.
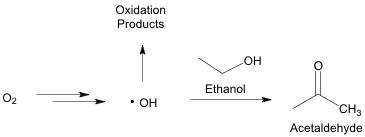
As has hopefully been made clear, using these reactions of acetaldehyde with tannins and anthocyanins is an important tool for winemakers. Winemakers can use several techniques to get the benefits of acetaldehyde on pigment and tannin structure. These use oxygen incorporation to form acetaldehyde through a series of metal-catalyzed reactions (Danilewicz 2003). Along this pathway, there is the possibility to form detrimental oxidation products instead of the desired acetaldehyde. A simplified version of this mechanism is shown in Figure 3. These other oxidation pathways can lead to some of the risks of oxygen exposure including the loss of desirable aromas, browning, and the formation of off odors.
Ideally, winemakers would be able to get the benefits of acetaldehyde without the risks of oxygen exposure. By adding acetaldehyde directly, we could avoid the problems of oxidation while simultaneously controlling the beneficial oxidation reactions we’re hoping for. With this in mind, we conducted an experiment where exogenous acetaldehyde was added during red wine fermentation using Cabernet Franc grapes from North East, PA.
The must was separated into three groups – control, low acetaldehyde, and high acetaldehyde. The acetaldehyde groups received four doses during the fermentation, 4×25 mg/L acetaldehyde in the low group and 4×250 mg/L acetaldehyde in the high group. Fermentations were performed in quadruplicate in microfermenters (Figure 4). Wines were fermented to dryness and pressed prior to analysis.
Wines were analyzed for color stability by the modified Somers assay (Mercurio 2007) with measures of sulfite-resistant pigments, or polymeric pigments, shown here. Astringency was measured using a model protein precipitation, where wines were mixed with bovine serum albumin (BSA) and the amount of tannin precipitated was quantified (Mercurio 2008). Higher tannin precipitation implies higher astringency. Data from this experiment is shown in Figure 5.
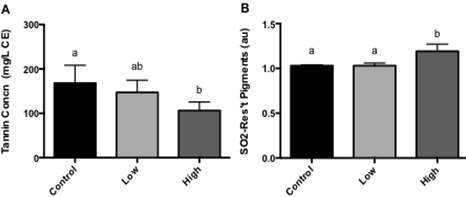
Figure 5. (A) Tannin content of BSA-tannin precipitates quantified by ferric chloride reaction in (+)-catechin equivalents. (B) Sulfite-resistant pigments in arbitrary units (au). Error bars represent one SD of the mean, and results with different letters (a,b) are significantly different (p < 0.05).
As shown in Figure 5, there was a statistically significant decrease in the measure of astringency and increase in color stability with high acetaldehyde treatment. This data provides evidence that exogenous acetaldehyde can be used in red wines to get beneficial effects on color and astringency without oxygen exposure. A more detailed discussion of the results can be found in the published manuscript (Sheridan 2015).
Based on this work, we are continuing to examine the reaction of acetaldehyde with tannins and anthocyanins. We are currently working with model wine experiments to further understand the chemistry of these reactions including characterizing the final products and the effect of wine composition.
Literature Cited:
Cheynier, V. & Dueñas-Paton, M. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305.
Danilewicz, J. C. Review of Reaction Mechanisms of Oxygen and Proposed Intermediate Reduction Products in Wine : Central Role of Iron and Copper. Am. J. Enol. Vitic. 2003, 54, 73–85.
Gambuti, A., Rinaldi, A., Ugliano, M. & Moio, L. Evolution of phenolic compounds and astringency during aging of red wine: effect of oxygen exposure before and after bottling. J. Agric. Food Chem. 2013, 61, 1618–27.
Mercurio, M. D., Dambergs, R. G., Herderich, M. J. & Smith, P. A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–7.
Mercurio, M. D. & Smith, P. A. Tannin quantification in red grapes and wine: comparison of polysaccharide- and protein-based tannin precipitation techniques and their ability to model wine astringency. J. Agric. Food Chem. 2008, 56, 5528–37.
Sheridan, M.K. & R.J. Elias. 2015. Exogenous acetaldehyde as a tool for modulating wine color and astringency during fermentation. Food Chem. 2015, 177, 17-22.
What contributes to red wine color? A primer on pigmentation in red wine.
By: Denise M. Gardner
Chemical Structure Figures By: Marlena Sheridan
Red wine color stability has been an important issue identified by the Pennsylvania wine industry during the 2013 and 2014 harvest seasons. It is likely to be a continuing issue for years to come. However, it should be noted that color stability issues are not localized to the Pennsylvania region.

Figure 1: Glass on left contains Chambourcin with deep color intensity while the glass on the right is Chambourcin wine with medium color intensity. Vintage 2013.
To comprehend problems affiliated with red wine color stability, an understanding of the components that are currently known to contribute to red wine color is essential.
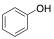
The phenol, a single benzene ring, is the chemical building block that contributes to many components affiliated in wine. Chemically, it is characterized by a hydroxyl group (-OH) attached to a benzene ring.
Two classes of phenolics, flavonoids and non-flavonoids, contribute to wine color. For the purpose of this discussion, we will focus on flavonoids, which are defined by containing 3 phenolic rings in their chemical structure, shown below.
There are several sub-classes within the flavonoid class that contribute to red wine pigmentation. This review will focus on 3 of these subclasses:
- Anthocyanins
- Flavan-3-ols (Catechins)
- Tannins (Proanthocyanidins)
Each one of these structures will contain the 3-phenolic ringed flavonoid backbone in their chemical structure (Figure 3). Variation of these subclasses exists with regards to attached units to the flavonoid backbone.
When reading the literature, understanding phenolic chemistry can become confusing, as many words like “tannins” can be used interchangeably with other terms (such as “proanthocyanidins,” “condensed tannins,” or “anthocyanogens”). This can be a daunting challenge for winemakers that are not proficient in chemistry-based terminology. Asking wine chemists for an explanation or clarification of terms is perfectly acceptable.
Monomeric Anthocyanins
Anthocyanins are the red pigment compounds, or chemical structures, that primarily contribute to wine color. Actually, anthocyanins can reflect a number of red-blue hues, often dependent on pH. There are many types of anthocyanins that are identified in grapes (e.g. malvidin, cyanidin, etc.) that are also present in other fruits. It’s important to note that much of the research to date is focused on malvidin-3-glucoside as it is the anthocyanin in highest concentration in Vitis vinifera species (with exception to Pinot Noir) and most easily accessible in a pure form, which makes it easy to obtain for research purposes.
However, many French-American hybrid varieties and native varieties (like Concord) typically contain a different anthocyanin profile in which malvidin-3,5-diglucoside may not be the primary anthocyanin (Ribéreau-Gayon 1959). Many hybrids and native varieties have a higher concentration of other diglucosides or acylated anthocyanins (Hrazdina and Franzese 1974, Ingalsbe et al. 1963). Acylated anthocyanins are often recognized for their contribution to “hybrid red wine character” as their expressed color falls in an orange to light red/pinkish hue (Mansfield 2015). [Note: the term “diglucoside” indicates that there are 2 (di-) sugar (-glucoside) units attached to the malvidin anthocyanin.]
Monomeric anthocyanins, or “free” anthocyanins, are noted for their susceptibility to bleaching by sulfur dioxide or to a change in hue due to a shift in pH. At wine pH (~3.4), only 10% of the monomeric anthocyanins exist in a state that expresses red color (i.e. the flavylium ion) (Somers and Evans 1974). Research has found that the majority of the monomeric anthocyanin exists in the hemiacetal structure, which is actually colorless (Kennedy et al. 2006). This is why anthocyanin concentration does not exactly reflect color intensity or color density of the wine.
Anthocyanin extraction occurs early in fermentation (Kennedy et al. 2006), and is usually at its maximum concentration by day 4 into primary fermentation (Mansfield 2015). Throughout the duration of primary fermentation, the concentration of monomeric anthocyanins will decrease by 10-20% due to insolubility in ethanol and adsorption by wine yeasts/lees (Kennedy et al. 2006).
Flavan-3-ol Monomers
Flavan-3-ols, also known as catechins, are found primarily in grape seeds (Kennedy et al. 2006). These components are large contributors to wine bitterness, but may also contribute to astringency at a lesser extent (Kennedy et al. 2006). In contrast to anthocyanins, monomeric flavan-3-ol concentrations tend to increase throughout the duration of primary fermentation (Kennedy et al. 2006). This is predominantly due to the increased allowable extraction time as seeds are in contact with the ethanol environment.
Tannins
Tannins, or proanthocyanidins, are polymers of favan-3-ol monomeric units. Tannins are responsible for the wine’s astringency, and are extracted from grape skins, seeds, and green matter (i.e. stems) (Kennedy et al. 2006). Tannins can also develop through the age of the wine by polymerizing monomeric favan-3-ols. Tannin extraction directly from the grape matter also appears to increase with longer skin/seed contact time during primary fermentation.
However, tannin extraction and utilization may also be variable in non-vinifera varieties. A recent study by Springer and Sacks (2014) from Cornell University found that many hybrid varieties actually contain higher concentrations of tannin-binding proteins inherent to hybrid variety’s disease- and cold-hardiness resistance. This could explain limited tannin extraction capabilities often affiliated with hybrid red wines. Additionally, if those proteins remain in the wine through primary fermentation, exogenous tannin additions may immediately bind to those proteins. Protein binding to exogenous tannins may minimize their chemical or sensory impact on hybrid wines.
Color Pigment Complexing: Copigmentation
Copigmentation describes an interaction between an anthocyanin and non-colored substrate (often referred to as a “copigment”) that enhances red-blue color hues of young red wines (Boulton 2001). Copigmented complexes are linked non-covalently. I like to think of this as the human equivalent of two individuals linking arms: the unit of 2 can still act as one, but can also easily separate.
The non-colored substrates affiliated with copigmented complexes include monomeric phenols (Figure 2), cinnamic acids, quercitin glycosides, vitexin, and orientin (Boulton 2001). Tannins play an extremely minor role in copigmentation (Boulton 2001).
It should be noted that some white grape varieties are believed to contain higher concentrations of non-colored substrates compared to many red wine varieties (Boulton 2001). Therefore, the act of blending 10-15% of a white grape variety (or white grape skins and seeds) to a red wine fermentation is believed to enhance the potential for copigmentation formation. Essentially, the concentration of co-factors is increased to encourage substrate availability for copigmentation. Malvasia with Sangiovese or Viognier with Syrah are classic white-red fermentation blends used in Old World winemaking regions to enhance red wine color. This phenomenon does not work if the white variety has insufficient concentrations of non-colored substrates (Boulton 2001).
Copigmented complexes are mainly affiliated with young red wines, as they tend to cause a shift in hue from red to a purplish-red. These complexes are less susceptible to sulfur dioxide bleaching or changes in wine pH compared to monomeric anthocyanins.
Color Pigment Complexing: Polymeric Pigments
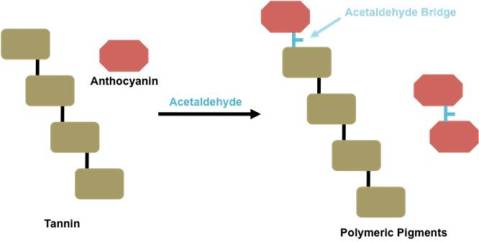
If the human equivalent of copigmentation complexes are two individuals linking arms, polymeric pigments can be thought of as two individuals each contributing one their individual legs into one pair of pants. This is because polymeric pigments are covalently linked anthocyanin-flavonoid complexes. (Recall that the term “flavonoid” can account for many other things such as another anthocyanin, tannin, etc.) Polymeric anthocyanin-anthocyanin pigments are known as vitisins (Kennedy et al. 2006).
Polymeric pigments can form under two different mechanisms. The first is through a direct linkage of an anthocyanin and another flavonoid substrate (Figures 4 and 5). In this case, the two units are covalently linked together. The image below details this using chemical structures and a schematic.
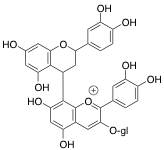
Figure 4: Chemical Structure Showing Direct Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation
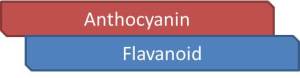
Figure 5: Schematic Picture Emphasizing Direct Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation
The second form of polymeric pigments is through acetaldehyde-bridged complexes (Figures 6 and 7). In this situation, acetaldehyde, an aroma compound typically associated with wine oxidation, forms a link, or bridge, between the anthocyanin and another flavonoid substrate. This is detailed below using chemical structures and a schematic.
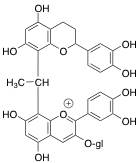
Figure 6: Chemical Structure Showing Indirect Acetaldehyde-Bridged Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation
Figure 7: Schematic Picturing Indirect Acetaldehyde-Bridged Covalent Linkage of Anthocyanin with Another Flavonoid Unit for Polymeric Pigment Formation
To make this topic even more confusing, the phenolic rings affiliated with anthocyanins and/or tannins can open and re-arrange within the polymeric pigment formation to form what is known as pyranoanthocyanins.
The advantage of polymeric pigment formation is that these complexes are not susceptible to sulfur dioxide bleaching or changes in wine pH. They are the most stable form of wine color known to scientists.
However, polymeric pigment formation tends to occur slowly in wine. Increased extraction of anthocyanins and tannins during primary fermentation offers available substrates to encourage polymeric pigment formation. However, increased extraction does not necessarily guarantee increased polymeric pigment formation.
Many winemakers add exogenous tannins at the beginning of primary fermentation in an effort to increase availability of tannins (or flavonoid substrates) when the anthocyanin concentration is highest. [Recall, anthocyanin concentration will decrease 10-20% by the end of primary fermentation.] This action is often referred to as the addition of “sacrificial tannins.”
However, not all exogenous tannins are created equally, or chemically react the same way in all wines. Additionally, many exogenous tannin products are not in a pure state (Mansfield 2015). Dr. Anna Katharine Mansfield recently reviewed phenolic wine chemistry and this concept in a 2015 Wines and Vine’s article titled: A Few Truths About Phenolics. For a more comprehensive understanding of phenolic chemistry, tannin structure (hydrolysed versus condensed tannins), and color stability, this article offers an easy read for any winemaker.
For winemakers looking to improve the color stability of hybrid varieties, remember that Springer and Sacks (2014) recently determined the high tannin-binding nature of many red hybrid varieties. This may indicate that tannin additions to red hybrid wines may not be useful.
Furthermore, lower anthocyanin concentrations (<200-300 mg/L catechin equivalents) may not be enough to drive the polymeric pigment reaction forward (i.e. create more polymeric pigments). A recent study by Sheridan and Elias (2015) investigate the polymeric pigment potential when acetaldehyde concentrations were increased during primary fermentation.
In order for polymeric pigment formation to occur, all three substrates are required at optimal concentrations (not clearly defined) of anthocyanins, other flavonoid units/tannins, and potentially acetaldehyde. This point alone may be a valuable indication for industry members to retain analytical records that measure the phenolic chemistry of incoming fruit and wines that have recently finished primary fermentation (prior to malolactic fermentation). Such records can act as indicators in terms of what further production methods may not be beneficial for red wine color enhancement. For example, a wine that is deficient in anthocyanin content (<200 mg/L) may not be a wine suitable for things like micro-oxygenation techniques that may contribute to polymeric pigment formation, but may benefit from potential co-fermentations with white varieties to enhance copigmented complexes.
In a future blog posts, we will explore the acetaldehyde-driven polymeric pigment reaction and how it influences red wine color stability. Additionally, this will be supplemented with a research-based literature review of common winemaking practices (e.g. cold soak, extended maceration, delayed MLF, etc.) that are commonly believed to enhance red wine color stability.
Literature Cited
Boulton, R. 2001. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 52(2): 67-87.
Kennedy, J.A., C. Saucier, and Y. Glories. 2006. Grape and wine phenolics: history and perspective. Am. J. Enol. Vitic. 57(3): 239-248.
Mansfield. A.K. January 2015. A few truths about phenolics. Wines & Vines.
Ribéreau-Gayon, P. 1959. Recherches sur les anthocyannes de vegetaux. Application au genre Vitis. Librairie general de l’enseignement, Pairs.
Sheridan, M.K. and R.J. Elias. 2015. Exogenous acetaldehyde as a tool for modulating wine color and astringency during fermentation. Food Chem. 177:17-22.
Springer, L.F. and G.L. Sacks. 2014. Protein-precipitable tannin in wines from Vitis vinifera and interspecific hybrid grapes (Vitis spp.): Differences in concentration, extractability, and cell wall binding. J. Agric. Food Chem. 62(30):7515-7523.
Somers, T.C., and M.E. Evans. 1974. Wine quality: Correlations with colour density and anthocyanin equilibria in a group of young red wines. J. Sci. Fd. Agric. 25:1369-1379.
Questions Pertaining to Malolactic Fermentation in Wine
By: Denise M. Gardner
What is Malolactic Fermentation?
Malolactic fermentation, MLF, is a bacterial fermentation, which converts malic acid to lactic acid. Malic acid, the acid affiliated with apples is perceptibly harsher than lactic acid, which is the primary acid in milk, and perceptibly softer than malic acid.
What is the difference between a native MLF and one that is inoculated?
MLF can occur spontaneously through the native microflora (i.e., lactic acid bacteria) that comes in on the fruit from the vineyard. Most native malolactic bacteria (MLB) strains are in the Lactobacillus genera. These bacterial populations are easily manipulated by pH changes and are sensitive to general environmental changes (e.g. increases in alcohol).
However, due to the fact that native or natural MLB is unpredictable and may contribute to several affiliated off-flavors of the wine, many winemakers choose to inoculate for MLF. The most common bacteria used for commercial inoculation is Oenococcos oeni, which can be purchased through a number of commercial suppliers. Many suppliers offer several different strain selections to accommodate varying wine conditions (e.g. high or low pH, high alcohol environments, use of sulfur dioxide, etc.). Commercial strains are bred to be reliable, consistent, and tolerant of low pH wines. They also contribute less flavor changes to the wine, if specific chains are selected by the winemaker, compared to many native bacterial strains.
One of the biggest problems affiliated with commercial MLF strains is the loss of color in red wines between primary and secondary fermentation. While we will touch briefly on color stability next week, several studies (Gerbaux and Briffox 2003, Morenzoni and Specht 2005, Burns and Osborne 2013) have noted the issues affiliated with red wine color stability since commercial strains have become more prevalent. Earlier this year, José Santos from Enartis Vinquiry discussed various red wine color stability issues during his “Harvest Preparation” workshop. You can read more about his discussion here.

Glass on left contains Chambourcin with deep color intensity while the glass on the right is Chambourcin wine with medium color intensity.
When should wines be inoculated for MLF?
Currently, inoculation and timing of inoculation for MLF is a stylistic decision made by the winemaker. There are several options available for any given wine:
- Following primary fermentation: Bacteria inoculation occurs after the completion of primary fermentation. This has become the standard way to use MLB.
- Co-Fermentation of yeast and bacteria: Bacteria inoculation occurs after inoculation of yeast for primary fermentation, but before primary fermentation is complete.
- Co-Inoculation or simultaneous inoculation: Here, yeast for primary fermentation and bacteria for MLF are inoculated at the same time to the must.
There are some advantages and concerns for co-fermentation or co-inoculation. In terms of advantages, previous research has highlighted how MLF bacteria can utilize remaining primary fermentation macromolecules (Guilloux-Benatier and Feuillat 1991), including mannoproteins (Alexandre et al. 2004) for their own nutritional gain. Beelman and Kunkee (1985) and King and Beelman (1986) found that bacteria acclimate to the changing wine conditions (i.e., increases in ethanol content) while the yeast strain proliferated to complete primary fermentation. Another advantage includes a reduction in total production time from the start of primary fermentation to the end of MLF, which can be beneficial to wineries with space or tank constraints.
In contrast, some research has pointed towards antagonistic relationships between yeast and bacteria, causing an increase in stuck fermentations. Typically, those studies also found an increase in volatile acidity. As mentioned previously, there has been an anecdotal observation of color intensity reduction in red wines, in general, utilizing commercial MLB strains. However, this observation has not yet been linked to timing of inoculation for MLF (Burns and Osborne 2013).
Based on the varying results from past research, it is advised that producers that utilize co-fermentation practices discuss strain selections, of both yeast and bacteria, with their supplier. There is a general review online, authored by Dr. Sibylle Krieger from Lallemand, written in 2007 regarding strain selection and MLF stylistic approaches, which you can read here.
Literature Cited
Alexandre, H. et al. (2004) Saccharomyces cerevisiae – Oenococcus oeni interactions in wine: current knowledge and perspectives. Int. J. Food Micro. 93: 141-154.
Beelman, R.B. and R.E. Kunkee. (1987) Inducing simultaneous malolactic/alcoholic fermentation. Practical Winery & Vineyard Management. July/August 1987. pg. 44-56.
Burns, T.R. and J.P. Osborne. (2013) Impact of Malolactic Fermentation on the Color and Color Stability of Pinot noir and Merlot Wine. Am. J. Enol. Vitic. 64:3, pg. 370-377.
Gerbaux, V. and C. Briffox. (2003) Influence de l’ensemencement en bacteries lactiques sur l’evolution de la couleur des vins de Pinot Noir pendant l’elevage. Revue des Înologues. 103:19-23.
Guilloux-Benatier, M. and M. Feuillat. (1991) Utilisation d’adjuvants d’origine levurienne pour améliorer l’ensemencement des vins en bactéries sélectionnées. Rev. Fr. Oenol. 132:51-55.
King, S.W. and R.B. Beelman. (1986) Metabolic interactions between Saccharomyces cerevisiae and Leuconostoc oenos in a model grape juice/wine system. Am. J. Enol. Vitic. 37(1): 53-60.
Morenzoni, R. and K. Scully Specht (Eds.). (2005) Malolactic Fermentation in Wine: Understanding the Science and the Practice. Lallemand Inc.
Getting Ready for Harvest – Part 4: Color Stability of Red Wines
By: Denise M. Gardner
The “Getting Ready for Harvest” seminar featuring José Santos from Enartis Vinquiry (California) included a detailed discussion about the technical aspects of several key processes that are currently of importance to Pennsylvania winemaking. Some of these topics included:
- White wine oxidation protection
- Yeast nutrition and its importance during fermentation
- Reducing “green” aromas and flavors in red wines
- Color stability in red wines
In this four part series, I will summarize some of the discussions led by José during the 2014 “Getting Ready for Harvest” workshop.
Stabilization of Color in Red Wines
The color of red wines exists in three forms:
- Free Anthocynanins
- Co-pigmented Anthocyanins
- Condensed Anthocyanins
Figure 1 describes these chemical differences and their influence on color stability. Free anthocyanins are most susceptible to color loss. This form is chemically unstable and sensitive to oxygen and sulfur dioxide. Co-pigmented anthocyanins are semi-stable forms of color pigments, which are anthocynanins that are weakly bound to tannins or polysaccharides (long-chain sugars). They are partially bleachable by sulfur dioxide. Condensed anthocyanins consist of covalently bonded anthocyanins to tannins, which elicits a strong bond. These forms of anthocyanins are color stable and are not bleachable by sulfur dioxide.
There are two time points within fermentation where a winemaker can focus on stabilizing red color:
- Until 2-4% alcohol is generated by primary fermentation
- In between primary fermentation and malolactic fermentation
The aqueous (i.e. water-based) environment prior to fermentation allows for tannin and anthocyanin extraction. Both components are needed to stabilize free anthocyanins. However, it is important to remember that more extraction does not guarantee better color stability. Tannin binding is non-specific, which means that tannins can bond to many different components found in wine including proteins or polysaccharides. All of these components are extracted during maceration. Once bound, the tannins are unavailable for anthocyanin bonding.
In this situation, the addition of sacrificial tannins may be required. Here the winemaker is making tannin additions prior to primary fermentation. Some of these added tannins will bind to other components within the fermenting must and precipitate out of solution during primary fermentation. As binding is nonspecific, some may also contribute to anthocyanin bonding interactions.
The last opportunity to stabilize color in red wines is in between primary fermentation and malolactic fermentation. At this stage, acetaldehyde generated during primary fermentation is the driving force to bind tannins and free anthocyanins. Oxygen is also needed to facilitate this reaction.
Therefore, winemakers are encouraged to use micro-oxygenation, or some incorporation of oxygen if micro-ox is unavailable, prior to the addition of malolactic bacteria to help facilitate condensation of anthocyanins. Additionally, winemakers should consider adding tannins, which increases the tannin concentration that could bind to anthocyanins. Regardless, winemakers should be incorporating oxygen to facilitate the anthocyanin-tannin bond.
Practicality in Production
The addition of tannins into the juice or must may seem counter-intuitive in cool climate regions that generate high-tannic red grapes on a regular basis. However, the basic building blocks to form condensed anthocyanins are needed to better stabilize red wine color, and increasing the starting pool of tannins allows for better bonding potential. Winemakers should acknowledge that not all tannins are equal, and some forms are better suited for specific functions. It is best if the winemaker contact their tannin supplier to determine which tannins are recommended for color stability purposes.
Color stabilization between primary and malolactic fermentation is important because after this period of time, sulfur dioxide is added to the wine. After malolactic fermentation has completed, most winemakers stabilize the microbial component of wine with sulfur dioxide. Sulfur dioxide binds readily with acetaldehyde, and once bound, the acetaldehyde cannot be used to fuel color stabilization reactions. Color stabilization reactions between tannins and anthocyanins occur at a much slower rate than sulfur dioxide binding with acetaldehyde.
Acetaldehyde is used as the connecting bridge between tannins and anthocyanins, which is why it is needed. Again, having more available tannins, which can be attributed by wine tannin additions, provides more potential building blocks for free anthocyanins to bind to. Using some sort of processing aid to integrate oxygen into the wine between primary and malolactic fermentation will help facilitate the binding reaction between tannins and anthocynains. Typically, oxygen addition is recommended using micro-oxygenation technology in which controlled rates of oxygen can be added to the wine. For those wineries that cannot afford a micro-ox investment, they can incorporate oxygen with pump-overs or splashing.